Tag Archives: thalassemia
Orphan Drug Exclusivity 2022 Best
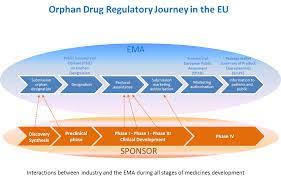
The aim of of this assignment is to identify another region or country (other than the US and EU) where Orphan Drug Exclusivity would be feasible and necessary for the secondary treatment of iron overload due to the treatment of beta thalassemia
Orphan Drug Exclusivity
Identify another region or country (other than the US and EU) where Orphan Drug Exclusivity would be feasible and necessary for the secondary treatment of iron overload due to the treatment of beta thalassemia. · Specify and explore the rationale as to why such secondary treatment qualifies or is eligible for Orphan Drug Designation (ODD). Define superiority for the drug candidate identified. · Present a high-level overview of the regulatory requirements for the approval of such secondary treatment under ODD.
Orphan Drug Exclusivity
Be sure to specify the prevalence of the population affected. Pediatric Exclusivity · Present a high-level overview of the regulatory requirements (laws and regulations) for the approval of such secondary treatment under pediatric exclusivity. · Be sure to discuss the regulatory mechanisms present within the region or country previously identified above. · Remember that the drug candidate must be superior to existing secondary treatments for iron overload due to the treatment of beta thalassemia within the three regions (US, EU, and _______)
Orphan Drug Exclusivity
Paper Requirements: The paper (2 pages) must fully and compellingly address the positions listed above. Proper grammar, spelling and citations are required. At least five (5) AMA (American Medical Association) citations are required. Please use standard margins, 1 inch, and Times New Roman, font size of 12. https://youtu.be/apwFXYJSKfE
Attached Files
|

 +1 650 405 4067
+1 650 405 4067

