Comprehensive clinical trial protocol 2022 Best
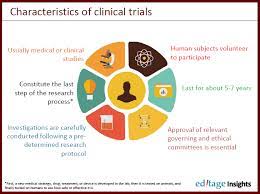
The goal of this template is to assist investigators to write a comprehensive clinical trial protocol that meets the standard outlined in the International Conference on Harmonization (ICH) Guidance for Industry
Comprehensive clinical trial protocol
The goal of this template is to assist investigators to write a comprehensive clinical trial protocol that meets the standard outlined in the International Conference on Harmonization (ICH) Guidance for Industry, E6 Good Clinical Practice: Consolidated Guidance (ICH-E6). Its use will also help investigators think through the scientific basis of their assumptions, minimize uncertainty in the interpretation of outcomes, and prevent loss of data. A common protocol structure and organization will facilitate protocol review by oversight entities. It is important to note that the clinical trial protocol template is just one piece of information required for an IND or IDE submission.
Comprehensive clinical trial protocol
For complete details on IND or IDE submissions see 21 CFR Part 312: Investigational New Drug Application or 21 CFR Part 812: Investigational Device Exemptions, respectively. How To Use This Template It is important to incorporate all sections of the template into your protocol and to do so in the same order. If a particular section is not applicable to your trial, include it, but indicate that it is not applicable. This template contains two types of text: instruction/explanatory and example. Instruction/explanatory text are indicated by italics and should deleted. Footnotes to instructional text should also be deleted.
Comprehensive clinical trial protocol
This text provides information on the content that should be included. It also notes if a section should be left blank. For example, many headings include the instruction, “No text is to be entered in this section; rather it should be included under the relevant subheadings below.” Example text is included to further aid in protocol writing and should either be modified to suit the drug, biologic or device (study intervention), design, and conduct of the planned clinical trial or deleted. Example text is indicated in [regular font]. Within example text, a need for insertion of specific information is notated by <angle brackets>.
Comprehensive clinical trial protocol
Instruction/explanatory text should be deleted. Example text can be incorporated as written or tailored to a particular protocol. If it is not appropriate to the protocol, however, it too should be deleted. The section headers include formatting to generate a table of contents. Version control is important to track protocol development, revisions, and amendments. It is also necessary to ensure that the correct version of a protocol is used by all staff conducting the study. With each revision, the version number and date located in the footer of each page should be updated. When making changes to an approved and “final” protocol, the protocol amendment history should be maintained (see Section 10.4). https://youtu.be/6WjI19bqH_8
Attached Files
|


 +1 650 405 4067
+1 650 405 4067

