The Grignard Reaction. 2022 Best
For today’s assignment we will focus on the Grignard Reaction. This post-lab assignment is a formal written laboratory report. Follow the guidelines below to write your report. You must type the report.
The Grignard Reaction.
Laboratory Assignment This post-lab assignment is a formal written laboratory report. Follow the guidelines below to write your report. You must type the report. Chemical structures should be drawn in ChemDraw. Scientific reports should be written objectively, in an impersonal past tense (e.g. “the chemical was added”, not “we added the chemical”). · Title: Your report should include a descriptive title (not “The Grignard Reaction”). · Names and Date: Include the names of all group members writing the report, and the date of the report. ·
The Grignard Reaction.
Purpose: The purpose should be concise (1 or 2 sentences) and state the objectives of the experiment, including the type of reaction studied, the material used/product obtained, and methods used to characterize the product or test for purity (i.e. melting point, TLC, NMR, etc.). In other words, why are you doing this experiment, and how are you doing it? · Chemical Reaction: Drawings of pertinent structures and/or reaction schemes.
Procedure/Observations: Observations (including color changes, exact masses, volumes, times, etc.) should be recorded throughout the experiment and included in the report with a summary of the procedure.
The Grignard Reaction.
Calculations: Any necessary calculations should be shown. For this experiment, that includes determination of the limiting reagent, calculation of theoretical yield of the product, and calculation of percent yield. Write out the relevant formula or equations and clearly show your work. Use proper significant figures and indicate proper units on all numbers for which units are applicable. · Results: This section should be a presentation of your data, and should include tables of data (as many as necessary), as well as a description of your products, including yield, appearance, and other physical properties.
The Grignard Reaction.
This section should not include any discussion. · Instrumental Analysis: This section should include any spectroscopic data obtained (NMR, IR, GC/MS, etc.), as well as any interpretation of this data. · Conclusions: This section should discuss the significance of your results, any conclusions you have reached based on your data, arguments to support those conclusions, and a discussion of the methods used to obtain your results. For this experiment, you should discuss the synthesis, separation, and purification techniques used.
The Grignard Reaction.
This section should also include conclusions about the purity of your product, the possible origin of any errors that you observed or that may lead to a poor yield or quality of products (including formation of unwanted by-products), and a comparison of experimental data and spectra to literature data. Do not include excess or irrelevant procedural detail. Procedure Week 1: Assemble the reaction apparatus shown on the photo page of this notebook section. Be sure that all of the glassware used in this experiment (including graduated cylinders) is clean and dry. Lightly grease all ground glass joints.
The Grignard Reaction.
Clamp a 100-mL single neck round bottom flask fitted with a Claisen connecting tube to a ring stand. Leave room for a water or ice bath under the flask. Do not support the flask on a ring. Place a reflux condenser in the side neck of the Claisen adaptor, and place a separatory funnel in the vertical neck. Fit a drying tube containing a small amount of glass wool and calcium chloride to the top of the condenser. Use blue Keck clamps at the appropriate joints. Attach clear Tygon tubing for water and start the flow of water. Make sure there are no leaks. Have the instructor check your apparatus.
The Grignard Reaction.
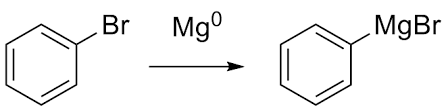
Grind 2 g of magnesium turnings in a mortar for a few minutes to provide a fresh surface. Place it in the 100-mL round bottom flask with 15 mL of anhydrous diethyl ether. Add 9 mL of bromobenzene to the flask. Warm the flask gently in the palm of your hand while gently grinding the Mg against the side of the flask with a glass stirring rod. Continue to warm the flask and use the stirring rod until the reaction starts, as indicated by bubbling and turbidity. (If your reaction does not start after a few minutes, it may help to use a starter solution.
Consult your instructor.) As soon as the reaction has started, reattach the flask to the connecting tube. https://youtu.be/U8ltC2Kz3Mk
Attached Files
|


 +1 650 405 4067
+1 650 405 4067

